Pipeline
Finding the 4%
At Algok Bio, we are laser-focused on building a healthier future by overcoming bottlenecks in today's drug development process. While only 4% of therapeutic candidates successfully complete the journey from discovery to market approval,1 we've identified a pivotal point in the R&D process where we believe that our successful drug development experience can move the needle toward success.
By identifying preclinical development-ready candidates that meet our criteria for success and bringing them through preclinical and clinical development, we can offer pharma partners lower-risk, high-value assets that are ready for licensing at either the late clinical development or post-approval stage.
Our featured asset below is just one example of the types of therapeutic candidates we are advancing. We look forward to expanding this pipeline by partnering with discovery teams looking for a development partner. We also encourage pharmaceutical licensing teams looking for promising assets to keep track of how our pipeline is progressing.
Our Featured Assets
Idetrexed
Small-molecule FRα-targeted inhibitor
IDOL Study: Combination Therapy (Idetrexed + Olaparib)
Indication: HGSOC (High-Grade Serous Ovarian Cancer)
Idetrexed + Olaparib
Monotherapy (Phase 2 Preparation)
Completed
Preparation
FRα: A Clinically Validated Target for Broad Oncology Indications
Folate Receptor alpha (FRα) is a highly promising and clinically validated target protein for cancer therapy. It is known to be overexpressed on the surface of various epithelial tumor cells—specifically in over 90% of ovarian and endometrial cancer patients and approximately 86% of TNBC patients—while exhibiting minimal expression in normal healthy cells. This makes FRα an ideal target for selective cancer treatment.
Lead Development Program: Idetrexed
Idetrexed is the only small-molecule FRα-targeted inhibitor that selectively accumulates in cancer cells overexpressing FRα. It works by inhibiting Thymidylate Synthase (TS), an essential enzyme for DNA synthesis, thereby blocking cancer cell proliferation.
Compared to other antifolate drugs, Idetrexed has a significantly lower binding affinity to the Reduced Folate Carrier (RFC), which is expressed in normal cells. As a result, it demonstrates superior selectivity for cancer cells with high FRα expression. Once absorbed by cancer cells, Idetrexed inhibits TS, leading to DNA synthesis blockade and inducing cancer cell death—all while sparing healthy cells and minimizing off-target toxicity. 2,3
Clinical Development Status and Key Results
Idetrexed successfully completed a Phase 1 clinical trial (CCR3941 Study) in ovarian cancer patients.4 The study enrolled a total of 109 patients and demonstrated a favorable safety profile. Most adverse events observed during the trial were mild and manageable, with no dose-limiting toxicities (DLTs) reported. Notably, Idetrexed did not exhibit the hematologic toxicity or vision-related side effects commonly associated with other Thymidylate Synthase inhibitors or FRα-targeted antibody-drug conjugates (ADCs), highlighting its superior safety profile.
In terms of efficacy, among platinum-resistant high-grade serous ovarian cancer (HGSOC) patients with moderate to high FRα expression, Idetrexed achieved an Objective Response Rate (ORR) of 36% (9 out of 25 patients). In contrast, the ORR in patients with low or no FRα expression was only 7.7%, clearly demonstrating that Idetrexed’s efficacy is highly dependent on FRα expression levels.
Given that current chemotherapy options for platinum-resistant ovarian cancer typically achieve an ORR of only 10–15%, the results from Idetrexed’s Phase 1 trial suggest it could offer a new and superior treatment option for these patients. Based on these promising findings, Algok Bio is preparing to initiate a Phase 2 clinical trial in the second half of 2025, exploring Idetrexed as a monotherapy for indications including endometrial cancer and TNBC.
Combination Therapy Strategy: Synergy with Olaparib (IDOL Study)
In addition to its potential as a monotherapy, Idetrexed has demonstrated strong synergistic effects when combined with other anti-cancer agents. Preclinical studies have shown that combining Idetrexed with Olaparib, a PARP inhibitor, enhances anti-cancer efficacy by more than 20-fold compared to Olaparib alone.
Building on this data, Algok Bio has partnered with the UK’s Institute of Cancer Research (ICR) to conduct the IDOL Study, a Phase Ib/II clinical trial evaluating the combination of Idetrexed and Olaparib. The trial aims to optimize combination dosing, assess safety, and establish early efficacy in ovarian cancer patients.
Publications & Posters
1. BioStock. (2023, January). Drug development: The four phases. Retrieved from https://www.biostock.se/en/2023/01/drug-development-the-four-phases
2. Gibbs, D. D., et al. (2005). BGC 945, a Novel Tumor-Selective Thymidylate Synthase Inhibitor Targeted to α-Folate Receptor–Overexpressing Tumors. Cancer Research, 65(24), 11721–11728. https://doi.org/10.1158/0008-5472.CAN-05-2034
3. Tochowicz, A., et al. (2013). Development and Binding Mode Assessment of BGC 945, a Novel Thymidylate Synthase Inhibitor that Targets Tumor Cells. Journal of Medicinal Chemistry. https://pmc.ncbi.nlm.nih.gov/articles/PMC3880649
4. Banerjee, S., et al. (2022). A Phase I Trial of CT900, a Novel α-Folate Receptor–Mediated Thymidylate Synthase Inhibitor, in Patients with Solid Tumors with Expansion Cohorts in Patients with High-Grade Serous Ovarian Cancer. Clinical Cancer Research. https://pmc.ncbi.nlm.nih.gov/articles/PMC9623233
AGK-102
Anti-TM4SF4 monoclonal antibody
AGK-102 is a candidate therapeutic antibody that we are developing as a precision medicine targeted at cells that express the novel cancer stem cell biomarker transmembrane 4 L six family member 4 (TM4SF4).
Cancer stem cells possess properties that make them hard to treat, including self‐renewal, metastasis, apoptosis, heterogeneity, immune resistance, and radio/chemoresistance. By pioneering development of a first-in-class cancer stem cell inhibitor, Algok Bio is opening the door to a novel method for treating cancers.
| Licensed Technology |
|
|---|---|
| Mechanism of action |
|
| Target Indications |
|
| Development Status |
|
Mechanism of Action
AGK-102 was developed by researchers at the Korea Atomic Energy Research Institute as a monoclonal antibody that specifically binds to the novel cancer stem cell biomarker TM4SF4 .
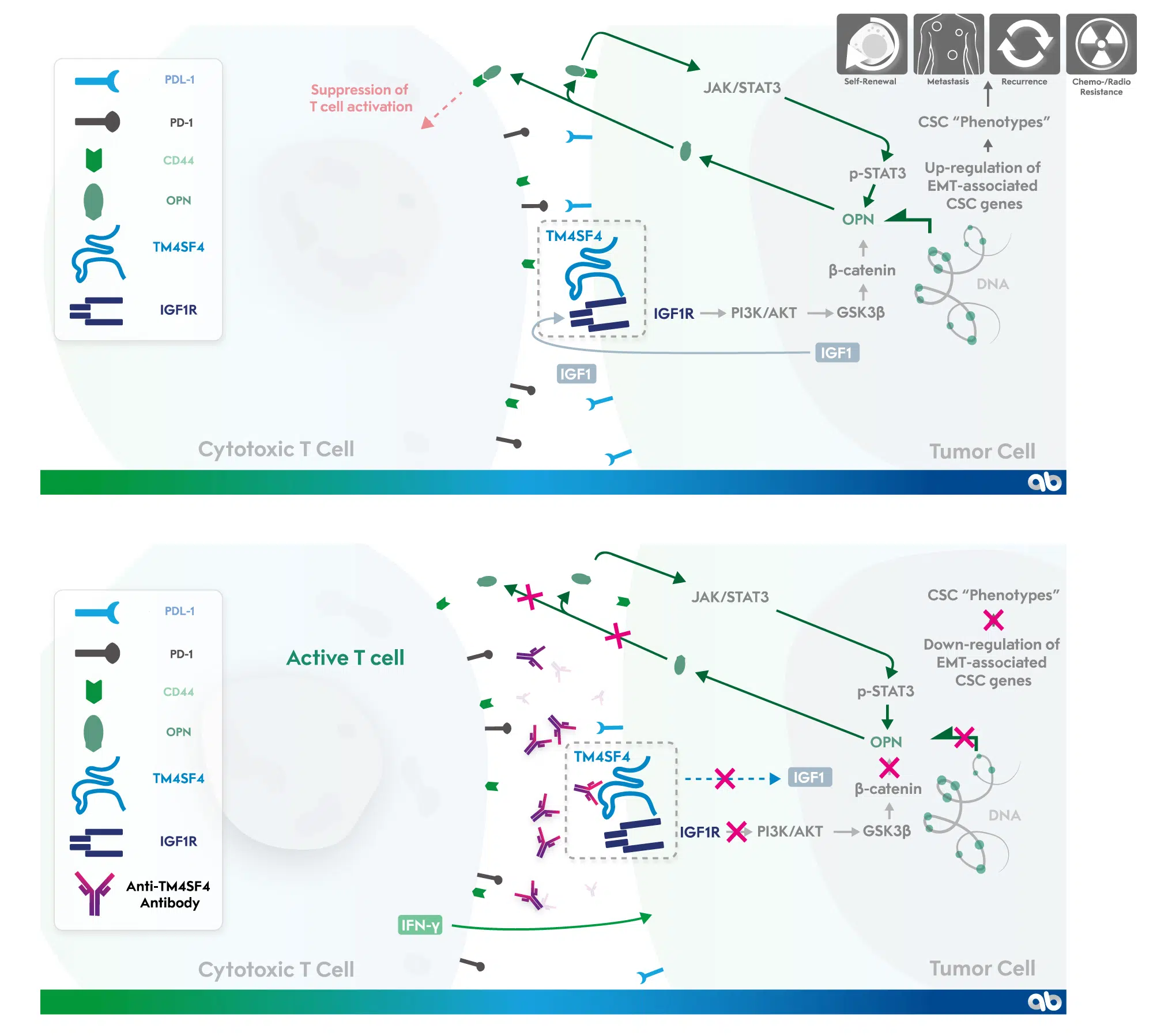
TM4SF4 is highly expressed in radiation-resistant lung adenocarcinoma cells and activates the IGF1R signaling pathway, which induces tumor progression.2 Importantly, TM4SF4 overexpression promotes epithelial-to-mesenchymal transition (EMT)-associated cancer stem cell (CSC)-like properties through induction of osteopontin (OPN) expression, which is known to be strongly correlated with poor prognosis in various cancer types. OPN reinforces the CSC-like properties through a positive feedback autocrine loop with the JAK2/STAT3 or FAK/STAT3 pathways, and also plays an immune-suppressive role in the tumor microenvironment. 3
AGK-102 binds to TM4SF4 resulting in the following:
- Down-regulation of IGF1 and OPN expression
- Interruption of the positive feedback autocrine loop between OPN and JAK/STAT3 pathway
- Activation of cytotoxic T-cells
- And, ultimately, suppression of CSC-like properties and tumor progression
Publications & Posters
1. BioStock. (2023, January). Drug development: The four phases. Retrieved from https://www.biostock.se/en/2023/01/drug-development-the-four-phases
2. Choi, S. I., et al. (2014). TM4SF4 overexpression in radiation-resistant lung carcinoma cells activates IGF1R via elevation of IGF1. Oncotarget, 5(20), 9823–9837. https://doi.org/10.18632/oncotarget.2450
3. Choi, S. I., et al. (2017). Osteopontin production by TM4SF4 signaling drives a positive feedback autocrine loop with the STAT3 pathway to maintain cancer stem cell-like properties in lung cancer cells. Oncotarget, 8(60), 101284–101297. https://doi.org/10.18632/oncotarget.21021
4. Kim, R. K., et al. (2024). A novel monoclonal antibody targeting TM4SF4 enhances antitumor activity through regulation of cellular levels of immune checkpoint ligands and antibody-dependent cellular cytotoxicity. Poster presented at the American Association for Cancer Research (AACR) Annual Meeting 2024. https://aacrjournals.org/cancerres/article/84/6_Supplement/3189/739837/Abstract-3189-A-novel-monoclonal-antibody

