Pipeline
Finding the 4%
At Algok Bio, we are laser-focused on building a healthier future by overcoming bottlenecks in today's drug development process. While only 4% of therapeutic candidates successfully complete the journey from discovery to market approval,1 we've identified a pivotal point in the R&D process where we believe that our successful drug development experience can move the needle toward success.
By identifying preclinical development-ready candidates that meet our criteria for success and bringing them through preclinical and clinical development, we can offer pharma partners lower-risk, high-value assets that are ready for licensing at either the late clinical development or post-approval stage.
Our featured asset below is just one example of the types of therapeutic candidates we are advancing. We look forward to expanding this pipeline by partnering with discovery teams looking for a development partner. We also encourage pharmaceutical licensing teams looking for promising assets to keep track of how our pipeline is progressing.
Our Featured Assets
Publications and Posters
References
1. Hingorani AD, Kuan V, Finan C, et al. Improving the odds of drug development success through human genomics: modelling study. Sci Rep. 2019;9(1):18911. doi:10.1038/s41598-019-54849-w
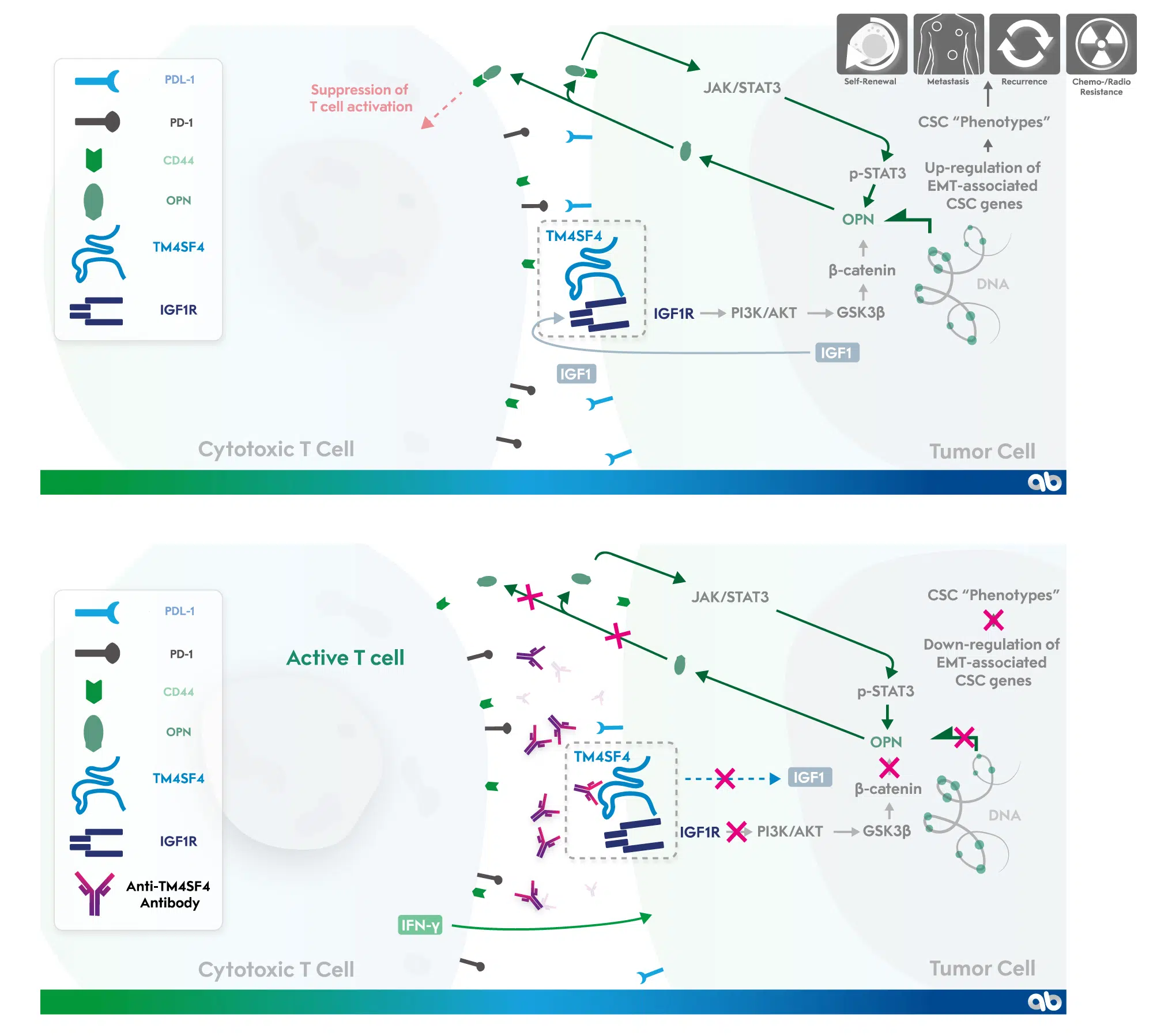
2. Choi SI, Kim SY, Lee J, Cho EW, Kim IG. TM4SF4 overexpression in radiation-resistant lung carcinoma cells activates IGF1R via elevation of IGF1. Oncotarget. 2014;5(20):9823-9837. doi:10.18632/oncotarget.2450
3. Choi SI, Kim SY, Lee JH, Kim JY, Cho EW, Kim IG. Osteopontin production by TM4SF4 signaling drives a positive feedback autocrine loop with the STAT3 pathway to maintain cancer stem cell-like properties in lung cancer cells. Oncotarget. 2017;8(60):101284-101297. doi:10.18632/oncotarget.21021